Boron Atomic Mass
The average atomic mass is can be calculated by the summation of the product of the atomic mass of each isotope multiplied by its abundance (abundance% / 100.0). Boron has two isotopes: 1) At. Mass = 10.012938 amu ≅ 10.013 amu and its abundance = 0.198 2) At. Amu and its abundance = 0.802 Average atomic mass of boron = 10.81 amu. Aug 06, 2019 Note that this is the value listed in the periodic table for the atomic mass of boron. Although the atomic number of boron is 10, its atomic mass is nearer to 11 than to 10, reflecting the fact that the heavier isotope is more abundant than the lighter isotope. Boron In 1961, the Commission recommended Ar (B) = 10.811 (3) based on calibrated mass-spectrometric measurements on brines and minerals from Searles Lake. The uncertainty was based on reported variations in natural abundances.
- Atomic Mass of Boron Atomic mass of Boron is 10.811 u.
- The mass number (symbol A, from the German word Atomgewicht atomic weight), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. The mass number is different for each different isotope of a chemical element.
Our editors will review what you’ve submitted and determine whether to revise the article.
Join Britannica's Publishing Partner Program and our community of experts to gain a global audience for your work!
What Is Boron Atomic Mass
The Editors of Encyclopaedia Britannica
Boron (B), chemical element, semimetal of main Group 13 (IIIa, or boron group) of the periodic table, essential to plant growth and of wide industrial application.
| atomic number | 5 |
|---|---|
| atomic weight | [10.806, 10.821] |
| melting point | 2,200 °C (4,000 °F) |
| boiling point | 2,550 °C (4,620 °F) |
| specific gravity | 2.34 (at 20 °C [68 °F]) |
| oxidation state | +3 |
| electron configuration | 1s22s22p1 |
Properties, occurrence, and uses
Pure crystalline boron is a black, lustrous semiconductor; i.e., it conducts electricity like a metal at high temperatures and is almost an insulator at low temperatures. It is hard enough (9.3 on Mohs scale) to scratch some abrasives, such as carborundum, but too brittle for use in tools. It constitutes about 0.001 percent by weight of Earth’s crust. Boron occurs combined as borax, kernite, and tincalconite (hydrated sodium borates), the major commercial boron minerals, especially concentrated in the arid regions of California, and as widely dispersed minerals such as colemanite, ulexite, and tourmaline. Sassolite—natural boric acid—occurs especially in Italy.
Boron was first isolated (1808) by French chemists Joseph-Louis Gay-Lussac and Louis-Jacques Thenard and independently by British chemist Sir Humphry Davy by heating boron oxide (B2O3) with potassium metal. The impure amorphous product, a brownish black powder, was the only form of boron known for more than a century. Pure crystalline boron may be prepared with difficulty by reduction of its bromide or chloride (BBr3, BCl3) with hydrogen on an electrically heated tantalum filament.
Limited quantities of elemental boron are widely used to increase hardness in steel. Added as the ironalloyferroboron, it is present in many steels, usually in the range 0.001 to 0.005 percent. Boron is also used in the nonferrous-metals industry, generally as a deoxidizer, in copper-base alloys and high-conductance copper as a degasifier, and in aluminum castings to refine the grain. In the semiconductor industry, small, carefully controlled amounts of boron are added as a doping agent to silicon and germanium to modify electrical conductivity.
In the form of boric acid or borates, traces of boron are necessary for growth of many land plants and thus are indirectly essential for animal life. Typical effects of long-term boron deficiency are stunted, misshapen growth; vegetable “brown heart” and sugar beet “dry rot” are among the disorders due to boron deficiency. Boron deficiency can be alleviated by the application of soluble borates to the soil. In excess quantities, however, borates act as unselective herbicides. Gigantism of several species of plants growing in soil naturally abundant in boron has been reported. It is not yet clear what the precise role of boron in plant life is, but most researchers agree that the element is in some way essential for the normal growth and functioning of apical meristems, the growing tips of plant shoots.
Pure boron exists in at least four crystalline modifications or allotropes. Closed cages containing 12 boron atoms arranged in the form of an icosahedron occur in the various crystalline forms of elemental boron.
Crystalline boron is almost inert chemically at ordinary temperatures. Boiling hydrochloric acid does not affect it, and hot concentrated nitric acid only slowly converts finely powdered boron to boric acid (H3BO3). Boron in its chemical behaviour is nonmetallic.
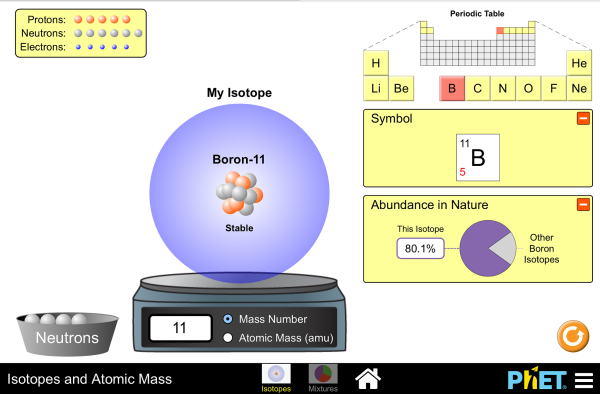
In nature, boron consists of a mixture of two stable isotopes—boron-10 (19.9 percent) and boron-11 (80.1 percent); slight variations in this proportion produce a range of ±0.003 in the atomic weight. Both nuclei possess nuclear spin (rotation of the atomic nuclei); that of boron-10 has a value of 3 and that of boron-11, 3/2, the values being dictated by quantum factors. These isotopes are therefore of use in nuclear magnetic resonance spectroscopy, and spectrometers specially adapted to detecting the boron-11 nucleus are available commercially. The boron-10 and boron-11 nuclei also cause splitting in the resonances (that is, the appearance of new bands in the resonance spectra) of other nuclei (e.g., those of hydrogenatoms bonded to boron).
The boron-10 isotope is unique in that it possesses an extremely large capture cross section (3,836 barns) for thermal neutrons (i.e., it readily absorbs neutrons of low energy). The capture of a neutron by a nucleus of this isotope results in the expulsion of an alpha particle (nucleus of a helium atom, symbolized α):
Since the high-energy alpha particle does not travel far in normal matter, boron and some of its compounds have been used in the fabrication of neutron shields (materials not penetrable by neutrons). In the Geiger counter, alpha particles trigger a response, whereas neutrons do not; hence, if the gas chamber of a Geiger counter is filled with a gaseous boron derivative (e.g., boron trifluoride), the counter will record each alpha particle produced when a neutron that passes into the chamber is captured by a boron-10 nucleus. In this way, the Geiger counter is converted into a device for detecting neutrons, which normally do not affect it.
The affinity of boron-10 for neutrons also forms the basis of a technique known as boron neutron capture therapy (BNCT) for treating patients suffering from brain tumours. For a short time after certain boron compounds are injected into a patient with a brain tumour, the compounds collect preferentially in the tumour; irradiation of the tumour area with thermal neutrons, which cause relatively little general injury to tissue, results in the release of a tissue-damaging alpha particle in the tumour each time a boron-10 nucleus captures a neutron. In this way destruction can be limited preferentially to the tumour, leaving the normal brain tissue less affected. BNCT has also been studied as a treatment for tumours of the head and neck, the liver, the prostate, the bladder, and the breast.
Boron Atomic Mass Formula
- key people
- related topics
Molar mass of B = 10.811 g/mol
Convert grams Boron to moles or moles Boron to grams
| Symbol | # of Atoms | Boron | B | 10.811 | 1 | 100.000% |
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.

A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
